3rd party supplement testing benefits for your brand


In the rapidly growing supplement industry, standing out requires more than just great products. While the FDA provides limited oversight of supplements, consumers demand proof of safety and efficacy. This is where 3rd party supplement testing becomes your brand’s competitive edge. By having your products independently tested, you verify ingredient claims, ensure product safety, and build the trust that turns first-time buyers into loyal customers.
For new and growing supplement brands, third-party testing isn’t an expense – it’s an investment in market leadership and sustainable growth. Let’s explore the concrete benefits this testing brings to your business.
Supplement testing importance
In the supplement industry, third party supplement testing is your brand’s first line of defense in ensuring product safety and reliability. While the FDA provides oversight, their monitoring happens only after products reach consumers – making proactive quality assurance crucial for your brand’s success and longevity in the market.
Regulatory reality:
- Supplements don’t need FDA approval before market launch.
- FDA checks begin only after products are on shelves.
- Regulatory action typically occurs only when problems are reported by consumers or competitors.
- Manufacturers are responsible for ensuring their products are safe before distribution.
Potential risks for untested supplements:
- The contents of the bottle may not match label claims, causing customer dissatisfaction and legal risks.
- Inconsistent product quality between batches can undermine brand reliability.
- Contaminants or adulterants may go undetected, posing safety risks and damaging brand reputation.
Benefits of third party testing
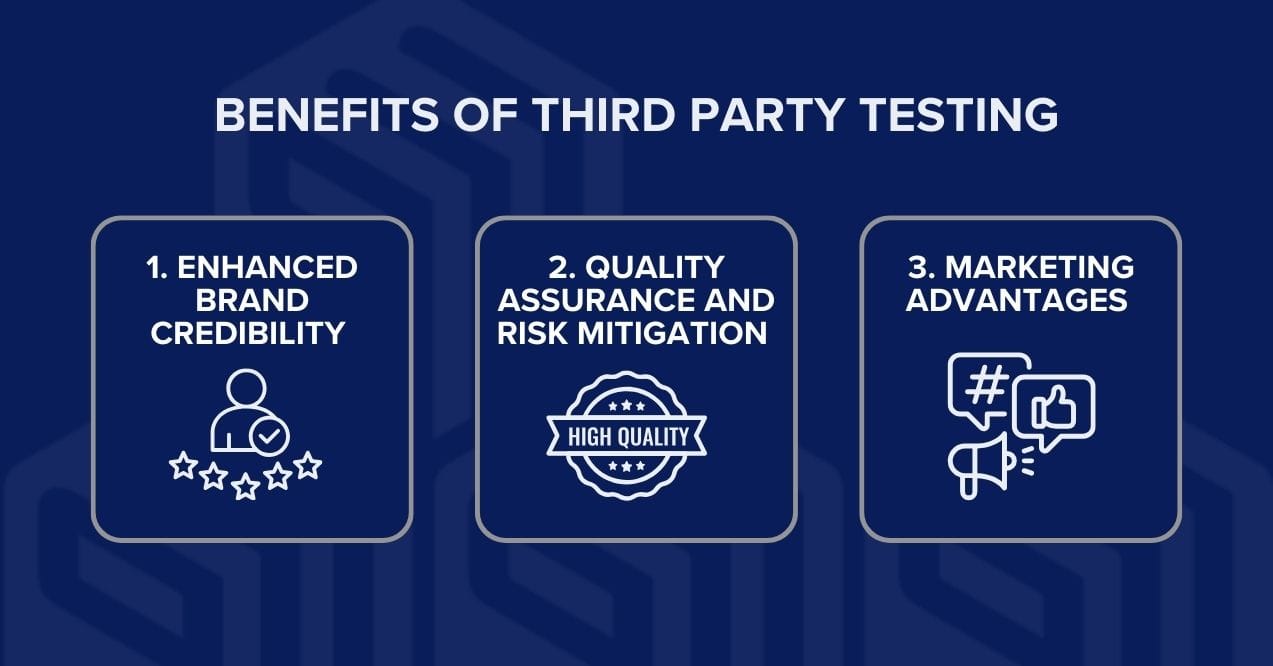
When you invest in third-party testing, you’re investing in your brand’s future success. While quality testing may seem like a significant expense initially, the benefits far outweigh the costs. Here are key advantages that make 3rd party supplement testing a smart business decision.
1. Enhanced brand credibility
In today’s saturated supplement market, transparency isn’t optional – it’s essential. When consumers see “third party tested supplements” on your label, it instantly signals your commitment to quality. This independent verification helps your brand stand out among competitors who might cut corners.
By choosing transparent testing practices, you’re not just selling supplements; you’re building trust and establishing your brand as a quality leader.
2. Quality assurance and risk mitigation
Implementing third party vitamin testing creates a robust quality control system that protects both your customers and your brand. Testing helps:
- Catch potential issues before products reach consumers
- Maintain consistent product quality across batches
- Document your commitment to safety standards
- Reduce the risk of costly recalls and reputation damage
3. Marketing advantages
Third-party testing provides powerful marketing opportunities that resonate with today’s health-conscious consumers:
- Use certification marks on packaging and marketing materials
- Share detailed test results with transparency-focused customers
- Leverage testing credentials in educational content
- Build customer loyalty through demonstrated quality commitment
The testing process explained

Understanding how third-party testing works helps you make informed decisions for your supplement brand. Here are four key stages of the testing process that ensure your products meet quality and safety standards.
Stage 1: Sample collection
The journey begins with proper sampling – a critical step that impacts all subsequent testing results:
- Randomized batch selection ensures unbiased testing
- Professional handling prevents sample contamination
- Proper storage conditions maintain sample integrity
- Chain of custody documentation tracks every movement
Stage 2: Laboratory analysis
Modern testing laboratories employ sophisticated methods to analyze your supplements:
- Mass spectrometry detects trace compounds with precision
- HPLC testing separates and quantifies ingredients
- Gas chromatography analyzes volatile components
- Microbial testing ensures product safety
- Heavy metals screening protects consumer health
Stage 3: Documentation and reporting
Testing culminates in comprehensive documentation that validates your product quality:
- Certificates of Analysis (CoA) verify label claims
- Detailed reports break down all test results
- Pass/fail assessments for each quality parameter
- Clear documentation for regulatory compliance
Stage 4: Implementation
The final stage involves putting test results to work:
- Update product labels based on verified content
- Adjust manufacturing processes if needed
- Support marketing claims with test data
- Maintain testing records for quality assurance
Standards and protocols in third party testing
Third-party testing laboratories follow strict standards and protocols to ensure accurate, transparent, and high-quality testing. These guidelines cover everything from laboratory procedures to staff training, equipment calibration, and data integrity, ensuring consistent and reliable results.
Common Standards and Protocols:
- ISO 17025 – The global benchmark for testing and calibration laboratories, outlining essential requirements for technical competence and quality assurance.
- GLP (Good Laboratory Practices) – A regulatory framework that upholds consistency and reliability in laboratory processes, covering study design, personnel training, equipment maintenance, and documentation practices.
Certifications and Accreditations:
- NSF (National Sanitation Foundation) International – Leading certification organization developing standards and testing protocols for food, water, and consumer goods
- USP (United States Pharmacopeia) – Establishes stringent guidelines to ensure the purity, potency, and quality of medicines, dietary supplements, and food ingredients.
- ISO Certification – Laboratories with ISO accreditation demonstrate compliance with international quality management and technical standards, reinforcing their credibility and trustworthiness.
Future of third-party testing
The landscape of supplement lab testing is rapidly evolving with technological advances:
- Artificial Intelligence and machine learning are streamlining testing processes, enabling faster and more accurate results while reducing human error.
- Blockchain technology is creating immutable records of test results that brands can share directly with consumers, setting new standards for transparency.
- Automation in sample handling and analysis is significantly reducing testing costs while improving consistency and throughput.
- Advanced analytics provide deeper quality insights, helping brands optimize formulations and maintain higher quality standards.
These innovations aren’t just improving testing accuracy – they’re making quality assurance more accessible and cost-effective for supplement brands of all sizes.
Final thoughts
As the supplement industry continues to grow, 3rd party supplement testing isn’t just an option – it’s a necessity for building a sustainable brand. By investing in independent testing, you’re not only protecting your customers but also securing your brand’s future in an increasingly competitive market.
Remember, consumers are more informed than ever, actively seeking products that prioritize transparency and quality. The initial investment in third-party testing pays dividends through enhanced brand credibility, reduced risks, and increased customer loyalty.
Take the first step toward strengthening your brand’s reputation by implementing a comprehensive testing program. Your customers – and your bottom line – will thank you.
It verifies supplement quality, ingredient accuracy, and freedom from contaminants through independent lab analysis.
Common certifications include: “NSF Certified for Sport” , “Informed Sport” , “USP Verified”, “BSCG Certified Drug Free”.
To get the most up to date list, it is best to check the USP website directly, as the verified products change. However, USP verifies a wide variety of vitamin and mineral supplements, and other dietary supplements. You can check their website by searching for “USP verified products”.
Yes, the FDA does regulate dietary supplements, but differently than drugs. They are regulated as foods, not drugs. This means that the FDA does not approve dietary supplements before they are marketed. However, the FDA does have post-market authority, meaning they can take action against unsafe or mislabeled products.
‘GMP’ stands for Good Manufacturing Practices. GMP certification indicates that a manufacturer follows established quality control guidelines. These guidelines help ensure that products are consistently produced and controlled according to quality standards. This helps to ensure the identity, purity, strength, and composition of dietary supplements.
A Certificate of Analysis (CoA) is a document issued by a qualified laboratory that verifies a product’s specifications. It provides detailed test results, confirming the identity, purity, and potency of the ingredients in a supplement. This document is a valuable tool for consumers seeking transparency and quality assurance.
References
Program, H. F. (2024). Questions and answers on dietary supplements. U.S. Food And Drug Administration. https://www.fda.gov/food/information-consumers-using-dietary-supplements/questions-and-answers-dietary-supplements
Program, H. F. (2005). Dietary Supplement Labeling Guide. U.S. Food And Drug Administration. https://www.fda.gov/food/dietary-supplements-guidance-documents-regulatory-information/dietary-supplement-labeling-guide
Consumer Law Lawyer for food and supplement labeling – Glancy LLP. (2020). Glancy Prongay & Murray LLP. https://www.glancylaw.com/practices/consumer-class-actions/food-and-supplement-labeling/
Good laboratory practice compliance | European Medicines Agency (EMA). (n.d.). European Medicines Agency (EMA). https://www.ema.europa.eu/en/human-regulatory-overview/research-development/compliance-research-development/good-laboratory-practice-compliance
Ramadan, A., Yasin, H., & Pektas, B. (2024). The role of artificial intelligence and machine learning in software testing. arXiv (Cornell University). https://www.researchgate.net/publication/383754178_The_Role_of_Artificial_Intelligence_and_Machine_Learning_in_Software_Testing
Strebinger, A., & Treiblmaier, H. (2024). Disintermediation of consumer services through blockchain? The role of intermediary brands, value-added services, and privacy concerns. International Journal of Information Management, 78, 102806. https://www.sciencedirect.com/science/article/pii/S0268401224000549
COMMONLY ASKED QUESTIONS
Advertisement. This site offers health, wellness, fitness and nutritional information and is designed for educational purposes only. You should not rely on this information as a substitute for, nor does it replace, professional medical advice, diagnosis, or treatment. If you have any concerns or questions about your health, you should always consult with a physician or other health-care professional. Do not disregard, avoid or delay obtaining medical or health related advice from your health-care professional because of something you may have read on this site. The use of any information provided on this site is solely at your own risk.